BD Veritor™ System - CLIA-waived for Rapid Detection of Flu A+B
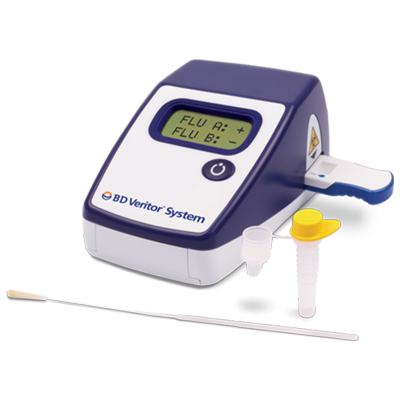
 INTENDED USE
INTENDED USE
The BD Veritor™ System for Rapid Detection of Flu A+B is a rapid chromatographic immunoassay for the direct and qualitative detection of influenza A and B viral nucleoprotein antigens from nasal and nasopharyngeal swabs of symptomatic patients. The BD Veritor System for Rapid Detection of Flu A+B (also referred to as the BD Veritor System and BD Veritor System Flu A+B) is a differentiated test, such that influenza A viral antigens can be distinguished from influenza B viral antigens from a single processed sample using a single device. The test is to be used as an aid in the diagnosis of influenza A and B viral infections. A negative test is presumptive and it is recommended that these results be confirmed by viral culture or an FDA-cleared influenza A and B molecular assay. Negative test results do not preclude influenza viral infection and should not be used as the sole basis for treatment or other patient management decisions. The test is not intended to detect influenza C antigens.
Performance characteristics for influenza A and B were established during January through March of 2011 when influenza viruses A/2009 H1N1, A/H3N2, B/Victoria lineage, and B/Yamagata lineage were the predominant influenza viruses in circulation according to the Morbidity and Mortality Weekly Report from the CDC entitled "Update: Influenza Activity - United States, 2010-2011 Season, and Composition of the 2011-2012 Influenza Vaccine." Performance characteristics may vary against other emerging influenza viruses.
If infection with a novel influenza virus is suspected based on current clinical and epidemiological screening criteria recommended by public health authorities, specimens should be collected with appropriate infection control precautions for novel virulent influenza viruses and sent to the state or local health department for testing. Virus culture should not be attempted in these cases unless a BSL 3+ facility is available to receive and culture specimens.
 ORDERING INFORMATION
ORDERING INFORMATION
| Product | Type | Sample Type | Kit Size |
| Rickettsia IgG/IgM Combo Test | Cassette | WB/S/P | 30 |
WORKING HOURS
- MON - FRI
- 8.00 AM – 05.00 PM
- SAT - SUN
- Closed
CONTACT DETAILS
 343/310 Khlonglamchiak Rd., Nuanchan, Buengkum, Bangkok, Thailand 10230
343/310 Khlonglamchiak Rd., Nuanchan, Buengkum, Bangkok, Thailand 10230 +662-1053447
+662-1053447 +662-1053448
+662-1053448 info@skmeditech.co.th
info@skmeditech.co.th +6692-260-4660
+6692-260-4660 www.skmeditech.co.th
www.skmeditech.co.th



